The surface of human skin has a naturally acidic pH in the range from 4-6, probably averaging 4.7. This acidic nature of skin is called the acid mantle and is vital for the proper functioning of skin. The low pH helps to protect the skin from bacterial infection, protects the barrier function of skin and helps the skin enzymes function properly. If the pH varies greatly, skin problems arise which may include infection, dehydration, dermatitis, roughness, acne, irritation, and noticeable flaking.
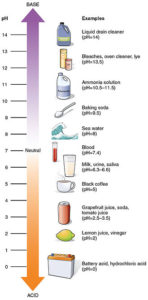 For a refresher, the pH refers to the concentration of hydrogen ion. It is a logarithmic scale that goes from 1-14 with 7 being neutral, less than 7 acidic and higher than 7 alkaline (basic). The pH of a solution can be measured with a pH meter, pH of skin is more difficult to measure and requires more specialized equipment. Read more here.
For a refresher, the pH refers to the concentration of hydrogen ion. It is a logarithmic scale that goes from 1-14 with 7 being neutral, less than 7 acidic and higher than 7 alkaline (basic). The pH of a solution can be measured with a pH meter, pH of skin is more difficult to measure and requires more specialized equipment. Read more here. What can affect the skin’s pH?
The most common thing that we do that can change the pH of the skin is cleansing the face with soap or other cleansers that have a high pH. Healthy skin can come back to its normal pH after a few hours, but not all skin can tolerate that challenge. Face toners are often used after cleansing to restore the skin pH back to acidic. Toners should always have an acidic pH for that reason. I like to use herbaldistillates/hydrosols for a toner. Modern face cleansers though are typically not soap and are buffered to be an acidic pH. Soap however has a basic pH of about 10 which cannot be changed, that may work great on most of your body, but if you have problems with skin on the face, do not use soap there. You can find a good face cleanser here that is pH balanced to skin.
People have often asked me about cleaning their face with baking soda. This is a big no no because baking soda has a high pH between 9-10 (similar to soap) and is sure to disrupt the pH balance of skin due to its alkalinity. Even plain tap water can affect skin pH. Theoretically, tap water should be pH 7, but it is typically closer to pH 8 because of impurities.
Age also affects skin pH, increasing as we age. For this reason it is important that products for mature skin have a pH from 4-5. Skin moisture, sweat, sebum, anatomic site, and genetic predisposition also affect skin pH.
The skin’s pH is maintained by secretions from the glands of the skin; both eccrine and sebaceous secretions.
Understanding the anatomy and function of skin can help you be a better formulator. If you would like to learn more, download our ebook “The Nature of Skin.”




Very interesting information! Thank you for sharing.
This is very interesting. I always wondered about the purpose of toner. Thanks for the info.